Menu
Rx Generic Product Catalog
Therapeutic Class: Oral Contraceptive
TE Code:
AB
AB
Therapeutic Equivalence [TE] Code:
Products meeting necessary bioequivalence requirements
OTC/Rx:
Rx
Rx
Requires a Prescription
Important Product Resources:
Including Black Box Warning
and Patient Labeling
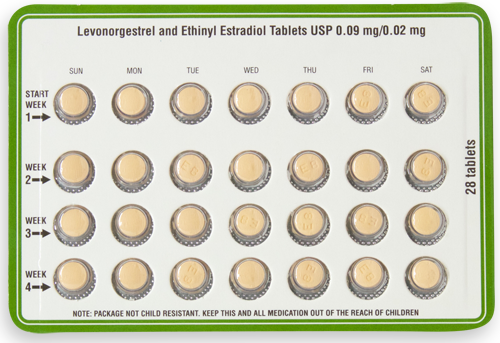
Strength: 0.09 mg/0.02 mg
Description:
Brownish Peach to Light Brown,
Round Tablets [Levonorgestrel and Ethinyl Estradiol]
Available Sizes [NDC & Pack]:
68462-0637-29
3 x 28 Carton
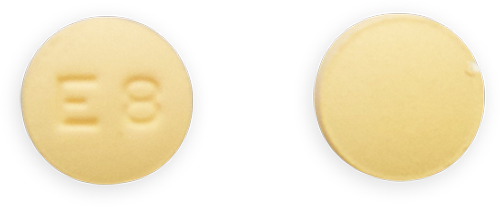
Imprint Side
1: E8
Imprint Side
2: Plain
Description:
Brownish Peach to Light Brown, Round Tablets
Brownish Peach to Light Brown, Round Tablets
Strength: 0.09 mg/0.02 mg
Contains:
Levonorgestrel and Ethinyl Estradiol
Levonorgestrel and Ethinyl Estradiol




